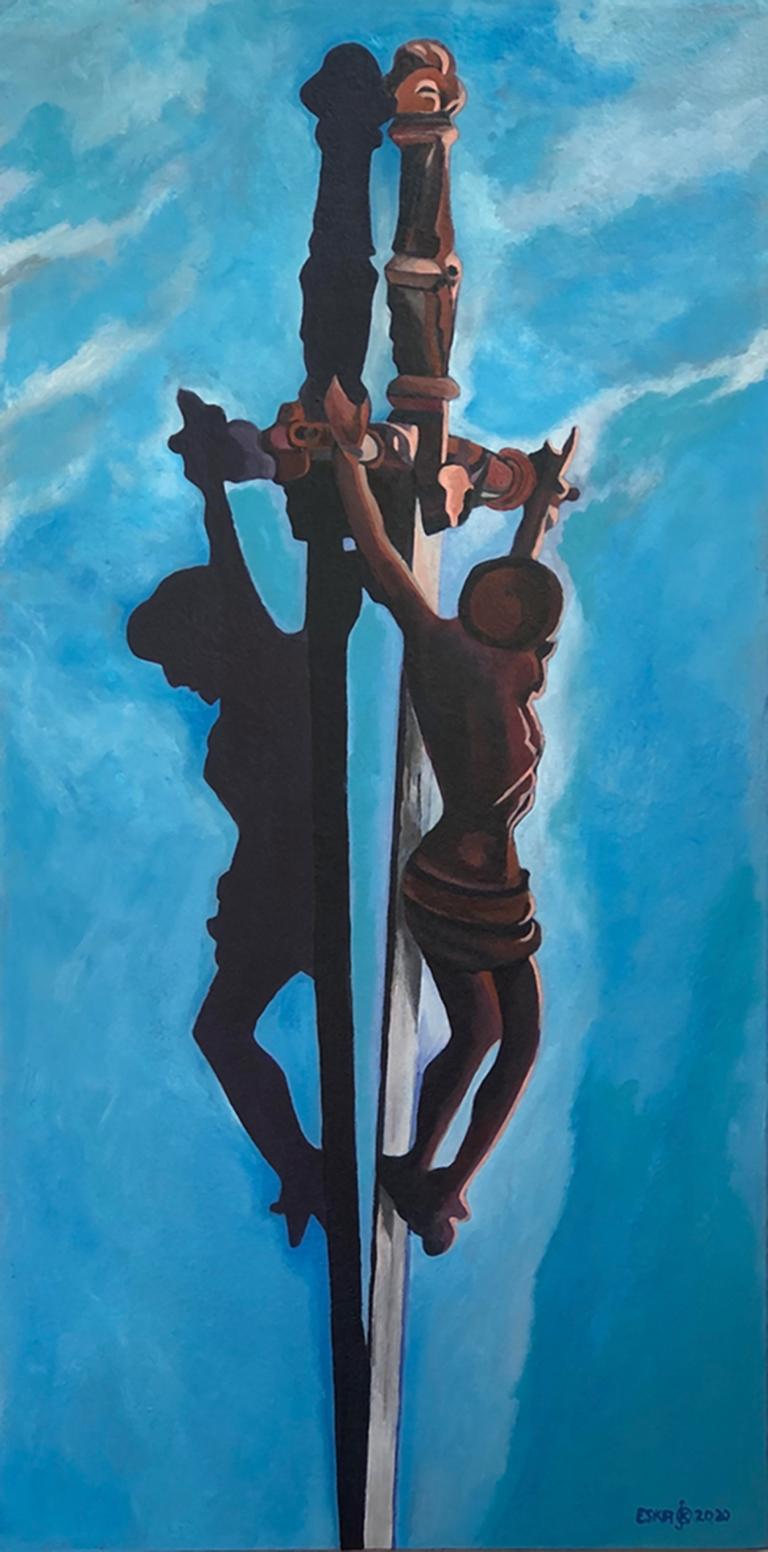Kunst und KultuR
von
Siegfried Knittel
Meine Malerei und meine Assemblagen versuchen Emotionen, denen auch autobiographische Bezüge zugrunde liegen, zu verarbeiten und zugleich auch dem Betrachter eigene Reflektionen zu ermöglichen. In unterschiedlichen Themen setze ich mich mit Architektur, Kreuzigungen, Friedenstauben, Angst und Unruhe und dem Schwarzwälder Bollenhut auseinander. Kunstprofessor Mathias Koeppel wertet meine Arbeiten als „Bildnerische Poesie zum Missverhältnis von Anspruch und Wirklichkeit.“
Siegfried Knittel
Sein Werk
Die Serie präsentiert Gebäude des Deutschen Bundestages, das Bundeskanzleramt und das Brandenburger Tor als Art von politscher Landschaft, in der frei von Ehrfurcht die Formensprache von Architektur in gestalterischer Analyse auflöst und in Farbflächen übersetzt. Die Farbe entwickelt eine Dynamik, die Heiterkeit, Dramatik aber auch Feuer oder gar Explosion symbolisieren könnten. Architektur und Politik werden mit Emotion verknüpft und eröffnen somit einen Interpretationshorizont
In dieser Kategorie befinden sich 43 Arbeiten
Die Gemälde offenbaren in explosiver Farbigkeit spontane seelische Stimmungen von Chaos, Unruhe, Angst und Emotion und dokumentieren diese in einen Geflecht von verworrenen Linien und Flächen. Für mich haben Gefühle wie Angst, Chaos und Unruhe kein Gesicht und keine Form und so sind diese Arbeiten in Hingabe bestimmter Stimmungen aus mir selbst in abstrakter Form spontan entstanden.
In dieser Kategorie befinden sich 51 Arbeiten
Sehen Sie Schwarzwaldmädchen, die man so noch nie gesehen hat. Neben Frauen aus allen Gesellschaftsschichten tragen auch berühmte Persönlichkeiten wie Maria Callas oder Simonetta Vespucci den Bollenhut und er steht ihnen sehr gut.
In dieser Kategorie befinden sich 90 Arbeiten
Diese Serie befasst sich mit der Friedenstaube als Symbol und versucht die Metamorphose ins Gegenteil als farbliche Emotionen darzustellen
In dieser Kategorie befinden sich 15 Arbeiten
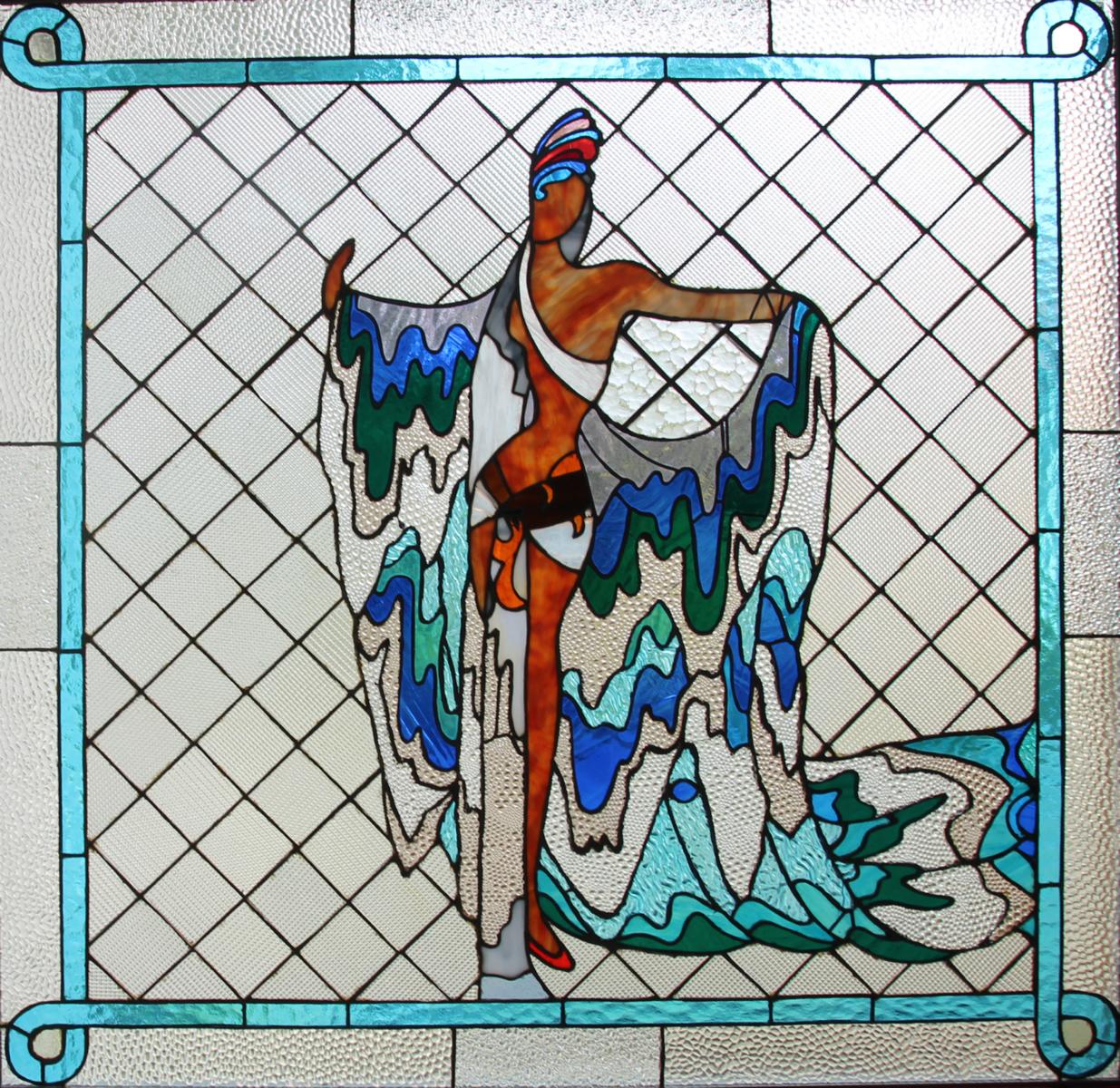
Die Glasobjekte als Mosaike und Glaskunst zeigen wie kein anderes Genre so leuchtende Farben und die Faszination von Licht.
In dieser Kategorie befinden sich 17 Arbeiten
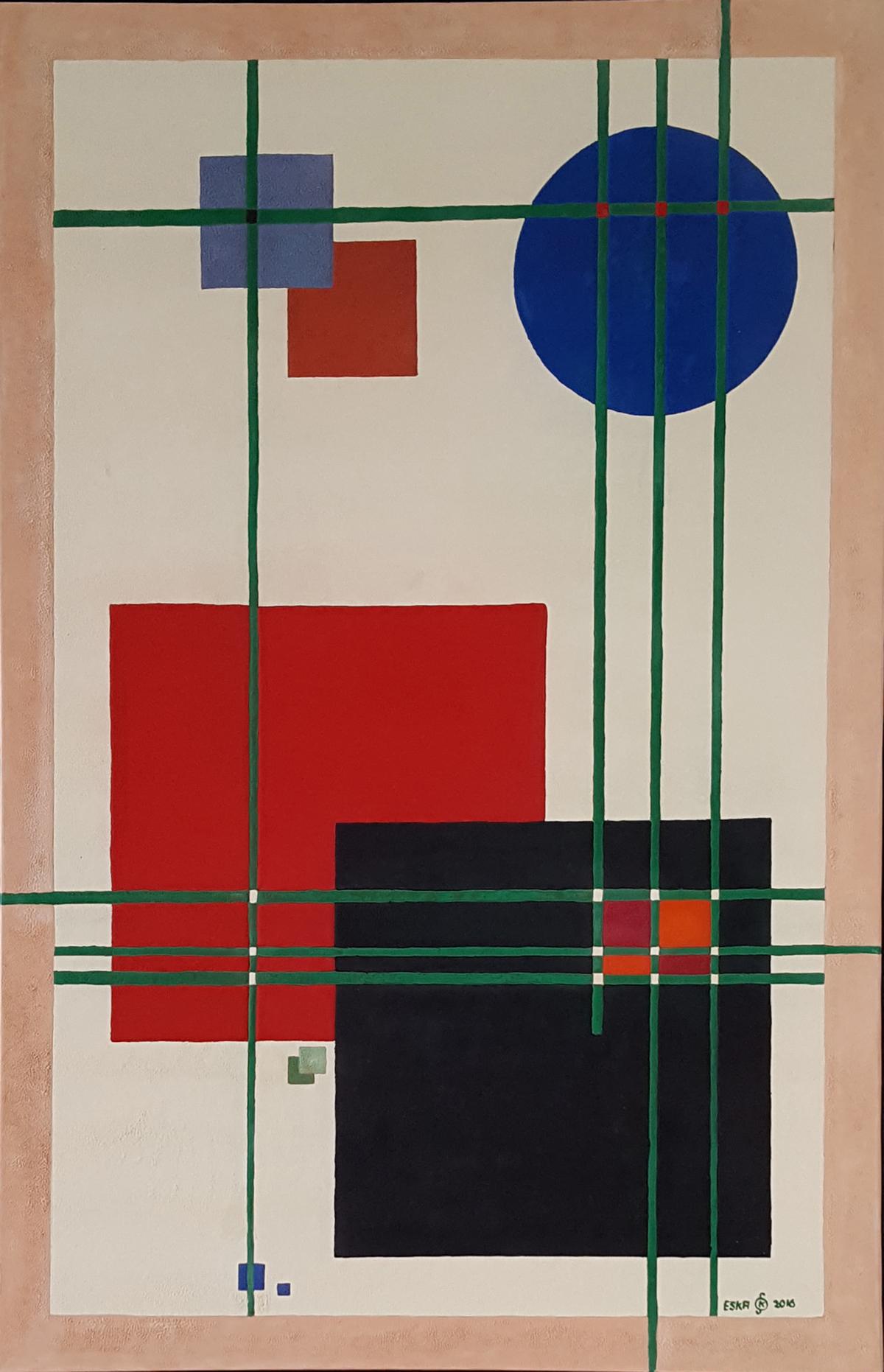
Die Gemälde zeigen im Gegensatz zur Abstrakten Malerei, geometrische Formen und Linien in einfachen, klaren Kompositionen und vermitteln Ordnung und innere Ruhe, als Gegenpol zum Chaos und versuchen insofern diese Polaritäten im Bild darzustellen.
In dieser Kategorie befinden sich 39 Arbeiten
Das Thema Kreuzigung ist von zentraler Bedeutung für mich. Das Kreuz als Solches ,ist ein universales Symbol, das in vielen Kulturen einen besonderen Stellenwert hat. Christus am Kreuz hingegen vermittelt ein christliches Symbol, das tief mit der gegensätzlichen Polarität von Mensch und Gott verwurzelt ist und existenzielle Fragen zum Glauben aufkommen lassen, deren Beantwortung jeder für sich finden muss.
In dieser Kategorie befinden sich 29 Arbeiten
Diese Uhrenobjekte versuchen den traditionellen Formen eine neue, modernere Variante zu geben indem Quadrat und Bollenhut als Stilelemente das formgebende Design prägen.
In dieser Kategorie befinden sich 6 Arbeiten